作者:汪盛
本文为作者授权医脉通发布,未经授权请勿转载。
免疫检查点抑制剂(ICIs)的出现极大改变了包括晚期肺癌在内的多个瘤种的治疗格局,并给患者带来显著生存获益。在此之前,晚期
然而,临床中只有部分患者从ICIs疗法中持续获益,寻找标准、可靠的生物标志物以筛选潜在ICIs获益人群仍是当前的研究重点。尽管PD-L1目前是临床实践中使用最多的生物标志物,但其对于ICIs治疗的预测价值仍存在争议[5]。其他预测性生物标志物正在探索中,包括肿瘤突变负荷(TMB)和基因组特征。
值得注意的是,最近的证据表明ARID1A基因的缺陷与免疫疗效相关。ARID1A是SWI/SNF染色质重塑复合物的一个重要组成亚基,在调节基因转录、细胞分化和器官发育等过程中具有重要的功能[6, 7]。ARID1A表达在NSCLC中被下调[8]。在超过50%的卵巢透明细胞癌,19%的
病例简介
患者,女,60岁,既往有吸烟史(35包/年)及
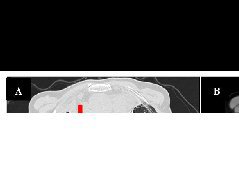
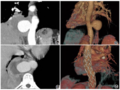
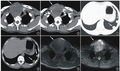
考虑到60%的PD-L1表达、极高的TMB和ARID1A突变,患者开始接受帕博利珠单抗单药治疗。5个月后,PET/CT图像显示肺病变(0.8cm)的摄取和大小显著降低,肾上腺肿块完全缓解。患者继续接受帕博利珠单抗单药治疗。

A-B:帕博利珠单抗治疗前;
C-D:帕博利珠单抗治疗5个月后。
讨论
免疫疗法彻底改变了NSCLC的治疗。不同的ICIs已被批准用于晚期NSCLC,无论是单药治疗还是与化疗联合治疗[11-13]。然而,只有一部分NSCLC患者从这些治疗中受益。在这种情况下,识别真正受益于免疫治疗的NSCLC患者以及确定预测效率更高的生物标志物至关重要。尽管大量生物标志物正在研究,但迄今为止,PD-L1表达是临床实践中免疫检查点抑制剂最常用的预测生物标志物。在接受ICIs治疗的晚期NSCLC患者中,较高的PD-L1表达与更好的应答率、无进展生存期(PFS)以及总生存期(OS)呈正相关[3]。然而,PD-L1是一种不完美的生物标志物,因为其在肿瘤上的动态表达以及存在抽样随机性的问题[14]。
最近,Hong等人发现来自肾上腺和肝转移灶的PD-L1比其他转移部位有更高的表达,并证明活检部位可以影响ICIs在NSCLC患者中的疗效;肺转移和远处转移PD-L1均与应答和生存相关,但淋巴结活检的PD-L1并不能预测临床获益[15]。一种新兴的生物标志物是TMB,高水平的TMB同样与更好的OS相关。当TMB ≥ 10 Muts/Mb时,与化疗相比,
ARID1A编码SWI/SNF复合物的一个亚基,该亚基对于控制基因表达至关重要,并与ICIs的疗效直接相关[18]。33086名NSCLC患者基因测序的结果显示,3.9%的患者存在至少1个功能性ARID1A的位点突变。考虑到在每个样本中检测到的所有变体,与没有ARID1A突变的样本相比,每个样本的ARID1A突变的中位改变数显著更高(P<0.0001),并且与腺癌相比,在
MSI是一种公认的免疫治疗生物标志物[22],ARID1A突变型的肿瘤比ARID1A野生型的肿瘤显示出更高的MSI [23]。Sarshekeh等人证明ARID1A与微卫星稳定
因此,本案例阐述了帕博利珠单抗单药成功治疗ARID1A突变型NSCLC 1例,并详细描述了患者的治疗效果及转归,供大家参考。
参考文献:
[1] Harada G, Amano MT, Antonacio FF, et al. Dramatic Response to Pembrolizumab Monotherapy in a Patient With ARID1A-Mutant Lung Adenocarcinoma: Case Report. Clin Lung Cancer. 2021;22(5):e708-e711.
[2] Noone AM HN, Krapcho M, Miller D, et al. SEER Cancer Statistics Review, 1975-2015. Bethesda, MD: National Cancer Institute; 2018 https://seercancergov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018.
[3] Garon EB, Hellmann MD, Rizvi NA, et al. Five-year overall survival for patients with advanced non–small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol. 2019;37:2518-2527.
[4] Gadgeel S, Rodriguez-Abreu D, Speranza G, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38:1505-1517.
[5] Grigg C, Rizvi NA. PD-L1 biomarker testing for non-small cell lung cancer: truth or fiction? J Immunother Cancer. 2016;4:48.
[6] Reisman D, Glaros S, Thompson EA. The SWI/SNF complex and cancer. Oncogene. 2009;28:1653-1668.
[7] Nagl Jr NG, Patsialou A, Haines DS, Dallas PB, Beck Jr GR, Moran E. The p270 (ARID1A/SMARCF1) subunit of mammalian SWI/SNF-related complexes is essential for normal cell cycle arrest. Cancer Res. 2005;65:9236-9244.
[8] Zhang Y, Xu X, Zhang M, et al. ARID1A is downregulated in non-small cell lung cancer and regulates cell proliferation and apoptosis. Tumour Biol. 2014;35:5701-5707.
[9] Jones S, Li M, Parsons DW, et al. Somatic mutations in the chromatin remodeling gene ARID1A occur in several tumor types. Hum Mutation. 2012;33:100-103.
[10] Hu G, Tu W, Yang L, Peng G, Yang L. ARID1A deficiency and immune checkpoint blockade therapy: from mechanisms to clinical application. Cancer Lett. 2020;473:148-155.
[11] Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823-1833.
[12] Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078-2092.
[13] Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016;27:559-574.
[14] Cottrell TR, Taube JM. PD-L1 and emerging biomarkers in immune checkpoint blockade therapy. Cancer J. 2018;24:41-46.
[15] Hong L, Negrao MV, Dibaj SS, et al. Programmed death-ligand 1 heterogeneity and its impact on benefit from immune checkpoint inhibitors in NSCLC. J Thorac Oncol. 2020;15:1449-1459.
[16] Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. New Engl J Med. 2018;378:2093-2104.
[17] Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. New Engl J Med. 2019;381:2020-2031.
[18] Yoh K, Matsumoto S, Furuya N, et al. Impact of SWI/SNF complex mutations in patients with non-small cell lung cancer (NSCLC) treated with immune checkpoint inhibitors: immuno-oncology biomarker study in LC-SCRUM-Japan (LC-SCRUM-IBIS). J Clin Oncol. 2020;38(15-suppl):9530.
[19] Gandara DR, Weipert C, Saam J, Riess JW, Lenz H-J, Kurzrock R. Prevalence and association of ARID1A with driver alterations and immune checkpoint inhibitor (ICPi) biomarkers in cell-free circulating tumor DNA (ctDNA) from 27,000 non-small cell lung cancer (NSCLC) patients. J Clin Oncol. 2020;38(15_suppl):9523.
[20] Shen J, Ju Z, Zhao W, et al. ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nat Med. 2018;24:556-562.
[21] Yang D, Xu Y, Huang L, et al. A pan-cancer analysis of ARID1A as a potential biomarker for immune checkpoint therapy. J Clin Oncol. 2020;38(15-suppl):3540.
[22] Chang L, Chang M, Chang HM, Chang F. Microsatellite instability: a predictive biomarker for cancer immunotherapy. Appl Immunohistochem Mol Morphol. 2018;26:e15-e21.
[23] Okamura R, Kato S, Lee S, et al. ARID1A alterations function as a biomarker for longer progression-free survival after anti-PD-1/PD-L1 immunotherapy. J Immuno Ther Cancer. 2020;8:e000438.
[24] Mehrvarz Sarshekeh A, Alshenaifi J, Roszik J, et al. ARID1A mutation may define an immunologically activesubgroup in patients with microsatellite-stable colorectal cancer. Clin Cancer Res. 2021;
- 上一篇:神经介入治疗带状疱疹引发尿潴留2例
- 下一篇:纳武利尤单抗成功治疗转移性卡波西肉瘤